Clinical research is necessary to establish the efficacy and safety of specific health products and medical practices. All the key changes, revisions and amendments in guidelines are designed to help clinical researchers and practitioners to protect human subjects and properly document trial results, maintain data quality and integrity.
Good Clinical Practice (GCP) Guidelines
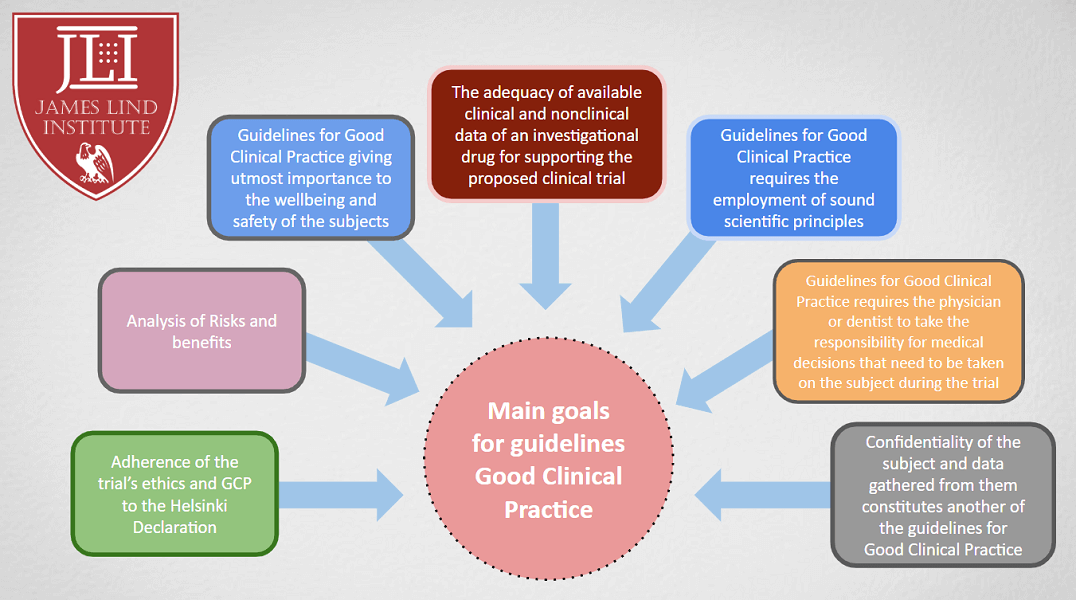
Clinical research processes relate to identify and explain activities relevant to health and medical products. The safety and efficacy information of specific products and treatments from clinical trials are tailored and designed to answer scientific and important healthcare questions. Trials have to be conducted according to standards and principles referred to as Good Clinical Research Practice (GCP). Established GCP guidelines incorporates scientific, ethical and quality standards for the designing, conducting, recording and reporting clinical research trials on human subjects. Compliance with GCP provides public assurance on protection of safety, rights and well-being of participating human subjects.
Guidelines for good clinical practice intends to assist sponsors, investigators, national regulatory authorities and ethics committees in implementing GCP for clinical research within industry, government agency or institutions. These guidelines continue to provide practical standardization for conducting clinical trials. Complex process of conducting clinical research is compounded by the involvement of different individuals with a variety of expertise skilled to perform tasks efficiently. These guidelines ensure the integrity of clinical research data by being consistent with the principles enunciated in the internationally recognized ‘Declaration of Helsinki’ and other ethical guidelines.
Requirement of Updating
Research has modernized and identifying concern areas for improvement is necessary to design and conduct clinical trials. Lack of harmonisation and revisions slows the adoption of innovative approaches in designing, managing, conducting, documenting and reporting clinical trials leading to inconsistency in approaches sponsors oversight, additional costing and time consuming process of developing drugs or medical products. Revisions without changing the core of the guidelines reflect on a modernized and evolving research landscape. Revisions and updating regulations focuses on increasing protection of human subject and data quality or integrity through better design of the trial study conducted. Revisions in guidelines aim to balance efficiency in clinical trials while protecting data integrity and retaining human subjects. Analysis of progress after implementation provides investigators and sponsors insights in areas that require further clarification. The working group consists of observers, industry experts and regulatory agencies that address and focus on current research topics of quality risk management, quality by design and new technological tools to ensure robust oversight in conducting and reporting trials.
Sponsors, investigators and other stakeholders in the research enterprise should be aware of the integrated addendum and new procedures in order to protect human subjects and ensure data integrity while designing and conducting clinical trials. The revised draft contains numerous additions and changes that address scale, complexity and cost of clinical trials in comparison to the previous version adopted. The update and addition in guidelines include changes made to clarify standards on electronic records and essential documents. New technology and risk management processes available to clinical researchers can increase efficiency and focus on relevant trial activities which are targeted in the guidelines. Amendments are made to encourage the implementation of more efficient and improved approaches to design, conduct and report clinical trials while ensuring data integrity and protecting participating human subjects.
Online Course at JLI
James Lind Institute (JLI) provides an online GCP Training & Certification Program to understand the importance of GCP in clinical research. For more information please visit: www.jli.edu.in